Borkin L. J., Korshunov A. V., Lada G. A., Litvinchuk S. N., Rosanov J. M., Shabanov D. A., Zinenko A. I. Mass occurrence of polyploid green frogs (Rana esculenta complex) in Eastern Ukraine // Russian Journal of Herpetology, 2004. – Vol. 11, No 3. – P. 194–213.
Mass occurrence of polyploid green frogs (Rana esculenta complex) in Eastern Ukraine
Leo J. Borkin,1 Alexey V. Korshunov,2 Georgiy A. Lada,1,3 Spartak N. Litvinchuk,4 Jury M. Rosanov,4 Dmitry A. Shabanov,2 and Alexander I. Zinenko5
1 Department of Herpetology, Zoological Institute, Russian Academy of Sciences, Universitetskaya nab. 1, St. Petersburg 199034, Russia. E-mail: lacerta@zin.ru
2 Department of Zoology and Animal Ecology, Kharkov National University, Kharkov, Svobody sq., 4, 61077, Ukraine. E-mail: d_sh@list.ru
3 Department of Zoology, Tambov State University, ul. Internatsional’naya, Tambov 392622, Russia. E-mail: esculenta@mail.ru.
4 Institute of Cytology, Russian Academy of Sciences, Tikhoretsky pr. 4, St. Petersburg 194064, Russia. E-mail: slitvinchuk@yahoo.com
5 Museum of Nature, Kharkov National University, Kharkov, Trinkler str. 8, 61022, Ukraine. E-mail: zinenkoa@yahoo.com.
Submitted August 20, 2004.
In eastern Ukraine, the Rana esculenta complex consists of three species: R. lessonae, R. ridibunda, and hybrid R. esculenta. The first one was rare, whereas two latter frog taxa were very common. Based on DNA flow cytometry, mass occurrence of the triploidy in Rana esculenta has been revealed in 14 localities of Kharkov, Donetsk, and Lugansk Provinces. One hybrid specimen from Kharkov Province was tetraploid. All polyploids were recorded along the middle part of Seversky Donets River (above 450 km). Triploids comprised two groups with different genome composition (LLR and LRR), and were found in three types of population systems (E, R–E, and L–E–R). Geographic distribution of polyploidy in European green frogs is briefly outlined. Different methods of ploidy level identification are discussed. The chromosome count and nuclear DNA cytometry provide the most reliable data.
Keywords: hybridogenetic frogs, triploidy, tetraploidy, Rana esculenta complex, DNA flow cytometry, Ukraine.
INTRODUCTION
Three taxa of European green frogs (Rana esculenta complex) are distributed in eastern Europe. These are two “normal” species – the lake frog, Rana ridibunda Pallas, 1771, and the pool frog, R. lessonae Camerano, 1882, as well as the edible frog, R. esculenta Linnaeus, 1758, which arose as a result of hybridization between the former two parental species. The complex exhibits an unusual mode of speciation, characterized by complicated genetic mechanisms, involving hybridization, so-called hemiclonal (or meroclonal) inheritance, polyploidy, unisexual and bisexual population systems of various kinds (e.g., Uzzell et al., 1977, 1980; Borkin et al., 1987; Vinogradov et al., 1988, 1990, 1991; Graf and Polls Pelaz, 1989a; Berger, 1990; Bucci et al., 1990; Günther, 1990; Tunner and Heppich-Tunner, 1991; Hotz et al., 1992; Caune and Borkin, 1993; Lada, 1995; Ragghianti et al., 1995). Rana esculenta is considered to be a klepton (in Greek: thief), a special category of taxa of the species group, the character of which does not coincide with that of the biological species (Dubois and Günther, 1982; Dubois, 1990; Günther, 1991).
Although hybridogenetic frogs (R. esculenta) are widely known across temperate Europe from France in the west to Volga River in the east (Günther, 1997; Borkin et al., 2003a, 2003b), natural triploidy has been recorded in many populations distributed in western and central parts of Europe only (e.g., Günther, 1970, 1975a; Knudsen and Scheel, 1975; Ebendal and Uzzell, 1982; Regnier and Neveu, 1986; Blommers-Schlösser, 1990; Plötner and Klinkhardt, 1992; Tunner and Heppich-Tunner, 1992; Berger and Berger, 1994; Mikulcek and Kotlík, 2001; Ogielska et al., 2001; Rybacki and Berger, 2001).
During two last decades, we examined by means of DNA flow cytometry numerous samples of green frogs from the territory of the former Soviet Union (Fig. 1). However, no record of triploidy in wild populations has not yet been published (Borkin et al., 1987; Vinogradov et al., 1988, 1990, 1991; Caune and Borkin, 1993; Lada et al., 1995; Borkin et al., 2003a, 2003b). An enigmatic tetraploid frog only was evidenced by karyotyping from Latvia (Borkin et al., 1979). An occasional triploid, taken by S. N. Litvinchuk from Kaliningrad Province, the westernmost enclave of Russia situated between Poland and Lithuania, has been mentioned by Borkin (2001).
In this contribution, we present the results of our study on green frogs of eastern Ukraine, obtained mainly on the basis of DNA flow cytometry. The aim of the paper is to give the evidence of mass occurrence of various kinds of polyploid frogs (R. esculenta) in local populations.
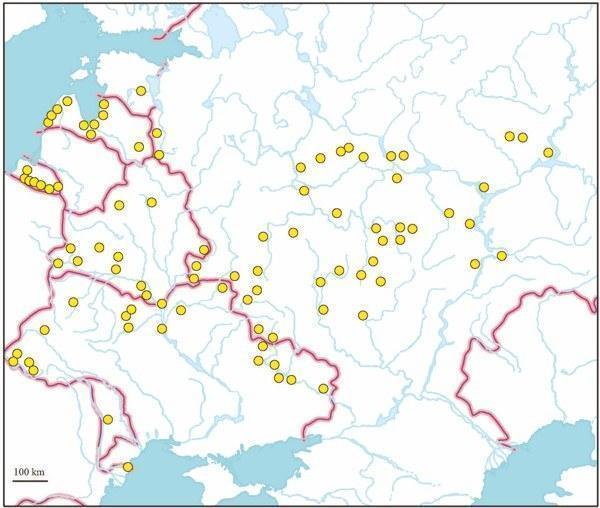
Fig. 1. The distribution of hybrid Rana esculenta in European part of the former Soviet Union (based on genome size data).
MATERIAL AND METHODS
Since 1989, a total of 551 green frogs were collected in eastern Ukraine and examined by DNA flow cytometry. In 1989 we studied 11 specimens from Lopan River, Kharkov City, presented by A. M. Rudik. In June 1995 we sampled 24 frogs from three other localities of Kharkov Province (Zmiyov and Kharkov Districts). In June 1996 we examined 43 specimens from two localities of Zmiyov and Zachepilovka Districts, Kharkov Province, Ukraine.
In 2002 – 2004 our sampling has been more extensive, and 473 frogs were taken from 38 localities of Kharkov, Donetsk, and Lugansk Provinces (Fig. 2, Table 1). The majority of specimens were collected at the “juvenile” stage, which covered both froglets before the first hibernation and subadults. Sex of adults, determined by dissection, and the age composition (juveniles or adults) of samples are represented in Table 1.
The frog catching by G. A. Lada has been selective in 1995 – 1996 and 2003 because he paid his attention to hybrids, and a few representatives of parental species were taken as a control and an evidence for their existence at a locality. However, Kharkov collectors sampled animals randomly, without any preference.
The amount of DNA per nucleus (genome size, in picograms, pg) was measured by DNA flow cytometry. The detals of the technique have been published previously (e.g., Vinogradov et al., 1990; Rosanov and Vinogradov, 1998; Borkin et al., 2001).
Population systems were identified by us using field observations and genome size data as well as, in a few cases, museum collections. Detailed analysis will be published elsewhere.
The species genome structure in frogs and the species composition in population systems are marked by the initial letter of species Latin names, namely: L is lessonae, R is ridibunda, and E is esculenta.
Frog samples are deposited in the collection of the Department of Herpetology, Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia, as well as in collection of the Museum of Nature, Kharkov National University, Kharkov, Ukraine.
The geographic names are given according to the map of Kharkov Province (Anonym, 1992).
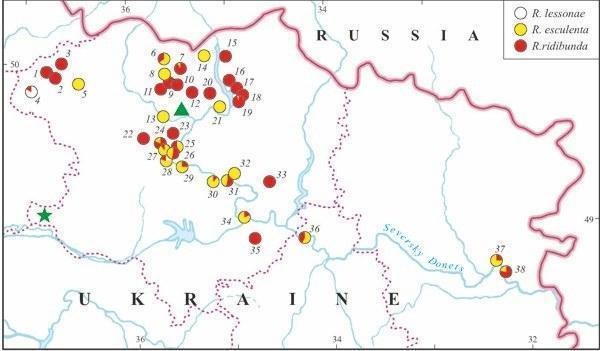
Fig. 2. The distribution of members of the Rana esculenta complex in eastern Ukraine (based on genome size data). The triangle marks the Kharkov City sample with R. ridibunda studied in 1989. The asterisk denominates the Russkii Orchik sample with three frog species studied in 1996.
TABLE 1. Genome Size Variation (in picograms) in Green Frogs collected from Eastern Ukraine in 2002 – 2004
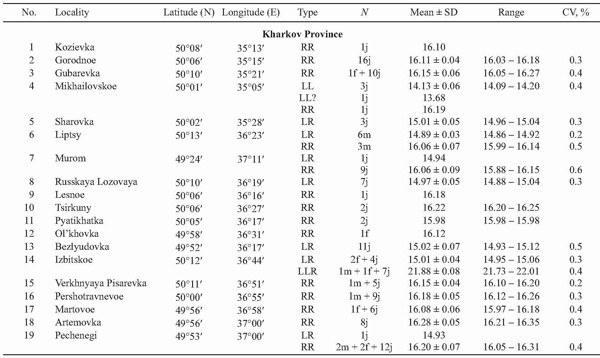

Fig. 3. Iskov Pond in the environs of the settlement Gaidary. The habitat of diploid and triploid Rana esculenta as well as R. ridibunda. Photo by Alexey V. Korshunov.
RESULTS
Species Genome Composition in Hybrids
According to genome size data, eleven water frogs of the first sample (1989) from Kharkov City (Fig. 2: marked by triangular) proved to belong to R. ridibunda.
Twenty four specimens taken from three localities in Kharkov Province (1995) were identified as R. esculenta. Five and 15 hybrids were collected from a pond in Koryakov Yar and from Iskov Pond (Fig. 3), respectively; both sites are situated in the environs of the settlement Gaidary (Fig. 2: the locality 26). Four frogs were captured in ponds of Ol'khovaya Balka Point, situated between villages Borshchevaya and Russkaya Lozovaya (Fig. 2, 8). All hybrids were diploid.
Another sample from Iskov Pond examined in 1996, contained R. esculenta and one male R. ridibunda, as it was evidenced by DNA flow cytometry. Among 36 hy- brids, we distinguished two groups: 35 diploids and one triploid (RRL).
Animals collected in 1996 from Russkii Orchik Point (Fig. 2, marked by asterisk), Zachepilovka District, contained representatives of three green frog species, namely: R. ridibunda (1), R. lessonae (1), and R. esculenta (4 diploids).
Thus, our preliminary studies made in 1989–1996 obviously indicated the occurrence of three members of the Rana esculenta complex in Kharkov Province, with numerous hybrids, including one triploid. These data did not included in Tables 1 – 4, since the nuclear DNA content was measured by DNA flow cytometry, with the use of different dye in comparison with our subsequent treatment, and because of different periods of sampling.
In 2002 – 2004 based on significantly more extensive sampling in three provinces of eastern Ukraine (473 specimens), we revealed six groups of animals expressed by different peaks on the DNA histogram (Fig. 4). We identified them as three members of the R. esculenta complex, namely: two parental species R. lessonae and R. ridibunda, and their hybrid R. esculenta (Tables 1–4).
The parental species were represented mostly by R. ridibunda (220 individuals or 46%), which had genome size values ranging from 15.74 to 16.35 pg. Only five specimens (or 1%) were reliably allocated to R. lessonae: their amount of nuclear DNA varied between 13.97 and 14.20 pg. Furthermore, one specimen from Mikhailovskoe (Fig. 2, 4), most likely, belonging to the same species, had lower amount of nuclear DNA in comparison with other R. lessonae (Tables 1 and 2). The ranges of genome size variation in both species did not overlap (Fig. 4, Table 2), and differences between averaged values reached 13.6%.
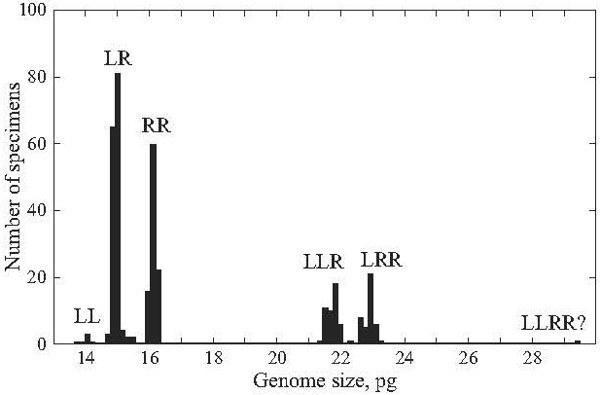
Fig. 4. The distribution of genome size in the Rana esculenta complex of eastern Ukraine according to species, ploidy level, and species genome composition. LL, R. lessonae; RR, R. ridibunda; LR, LLR, LRR, and LLRR?, four groups of hybrid R. esculenta.
TABLE 2. Genome Size Variation (in picograms) in Parental and Hybrid Species of the Rana esculenta Complex in Eastern Ukraine (N = 473)
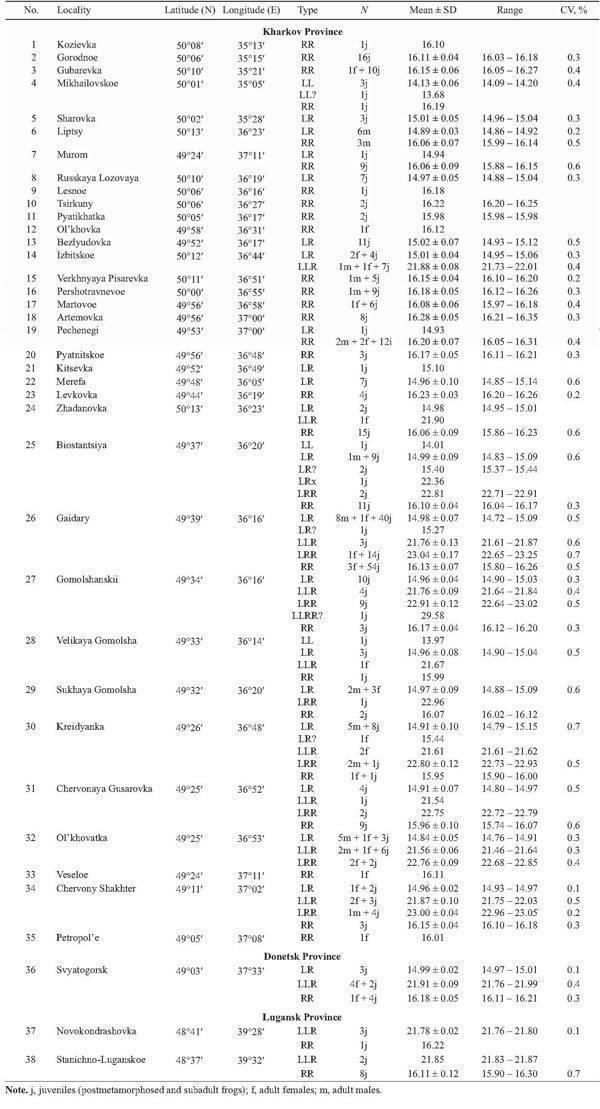
The majority of frogs under the study (247 specimens, or 52%) were assigned to R. esculenta. These hybrids produced four peaks associated with various ploidy and parental species genome composition (Fig. 4). Diploid frogs (LR), males and females, were intermediate between R. lessonae and R. ridibunda, and their genome size values were equal to 14.72 – 15.15 pg. Four specimens had somewhat higher value (15.27 – 15.44 pg) at the diploid level (Table 2). Thus, the diploid class provided almost 64% out of R. esculenta, examined by us in 2002 – 2004 (Figs. 5 and 6).
Among hybrids, the share of polyploid individuals was equal to 36% (n = 89). According to genome size (Fig. 4, Table 2), they were composed of 88 triploids (21.46 – 23.25 pg) and one tetraploid specimen (29.58 pg).
Triploid hybrids formed two groups (Figs. 4, 7, and 8; Table 2) having a little different animal numbers. The group (n = 46, or 52% of all polyploids) with lesser genome size was assigned to the triploid class with the LLR genome composition, i.e., two lessonae genomes and one ridibunda genome, because its averaged value (21.77 pg) was in agreement with theoretically expected estimate (L + LR → 7.035 + 14.96 = 22.00 pg). Another group (n = 41, or 46% of all polyploids) with larger genome size (22.93 pg) seemed to have the LRR genome structure, i.e., two ridibunda genomes and one lessonae genome, respectively (R + LR → 8.06 + + 14.96 = 23.02 pg). Genome size values in both triploid groups ranged without overlap. However, one triploid had an intermediate position between the both groups (LRx; Table 2).
Both kinds of triploids were represented by males and females.
A sole tetraploid specimen (an juvenile) showed the genome size value (29.58 pg) between theoretically expected values for hybrids with symmetrical ge- nome structure, LLRR (LL + RR → 14.07 + 16.12 = 30.19 pg) and for frogs of the LLLR genome type (LLL + R → 21.11 + 8.06 = 29.17 pg).
The genome size variation in all, both diploid and triploid, groups were quite similar: values of the coefficient of variation (CV) ranged from 0.5 to 0.7% (Table 2).
We failed to observe any correlation between sex or age (adults or juveniles), on the one hand, and the genome size variation, on the other hand.

Fig. 5. A diploid male of Rana esculenta from Liptsy, Kharkov Province. Photo by Spartak N. Litvinchuk

Fig. 6. A diploid male of Rana esculenta from Biostantsiya, Kharkov Province. Photo by Spartak N. Litvinchuk.

Fig. 7. A triploid female of R. esculenta with the LLR genome composition from Kreidyanka, Kharkov Province. Photo by Dmitry A. Shabanov and Alexey V. Korshunov.

Fig. 8. A triploid male of R. esculenta with the LRR genome composition from Kreidyanka, Kharkov Province. Photo by Dmitry A. Shabanov and Alexey V. Korshunov.
Green Frog Composition in Localities
The majority of localities, examined in 2002 – 2004, under the study was inhabited by R. ridibunda or, in lesser extent, by R. esculenta, whereas R. lessonae was recorded in three localities only, 8% (Fig. 2, Tables 1 and 3). Formerly, in 1996, the latter species was found in Russkii Orchik as well (Fig. 2). Twenty two localities were shared by two or three species of green frogs. We also found one locality (Mikhailovskoe) where both parental species were recorded together without hybrids. However, we could suggest that such system existed in other locality as well (Gorodnoe). Our samples from this locality, examined by DNA flow cytometry, were represented by R. ridibunda only. Nevertheless, we identified another species, R. lessonae, in the collection of the Museum of Nature, Kharkov University. In contrast, R. esculenta coexisted with R. ridibunda in 13 localities, whereas the occurrence of all members of the R. esculenta complex in the same localities was registered in two cases (Table 1). Moreover, all three members of the complex were recorded in Russkii Orchik (Fig. 2). Some localities (larger samples with ten or more specimens) were represented by one species only: either by R. ridibunda (3 cases) or by R. esculenta (3 cases).
Triploids were found in 14 localities (37%) of Kharkov, Donetsk, and Lugansk Provinces (Fig. 9). Their occurrence was associated with R. ridibunda in 11 cases, whereas both parental species coexisted with triploids in two localities only (Biostantsiya and Velikaya Gomolsha — Fig. 10; Tables 1 and 4). Samples from two localities (Bezlyudovka and Izbitskoe) contained diploid and triploid hybrids only, without any parental species.
Unlike diploid R. esculenta, triploids were recorded along the middle part of Seversky Donets River only. The most distant localities with triploids were separated by the distance of above 450 km (from Izbitskoe village to Stanichno-Luganskoe village — Fig. 9).
A tetraploid frog was found in the locality Gomolshanskii, Kharkov Province (Fig. 9, Table 1 and 4) where both kinds of triploids co-existed with parental species.
Triploid Genom Structure and Population Systems
Triploids of the LLR composition only were revealed in three types of population systems (Table 4), namely: in the E type (for instance, Izbitskoe), the R-E type (for instance, Svyatogorsk, Novokondrashovka, and Stanichno-Luganskoe), as well as in the L-E-R type (for instance, Velikaya Gomolsha).
Triploids with the LRR genome structure only were found in the L-E-R type of population systems (Biostantsiya and Sukhaya Gomolsha — Table 4).
Both kinds of triploids together were revealed in two types of population systems (Table 4). These were mixed populations of the R-E type (for instance, Kreidyanka — Fig. 11, and Ol'khovatka) and the L-E-R type (for instance, Gaidary).
TABLE 3. Frequency of Green Frog Species in Localities of Eastern Ukraine (population systems are based on field, museum and genome size data)

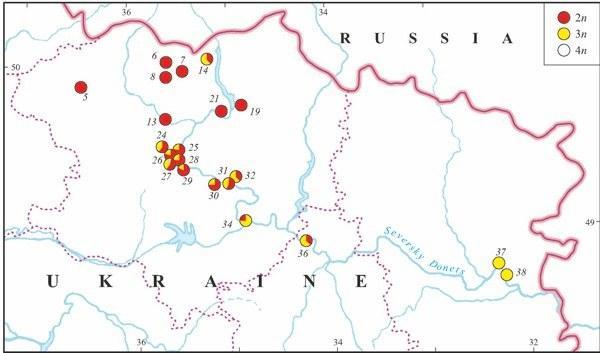
Fig. 9. The distribution of diploid and polyploid hybrids in eastern Ukraine (based on genome size data).
TABLE 4. Incidence of Diploid and Triploid Hybrids in Localities of Eastern Ukraine
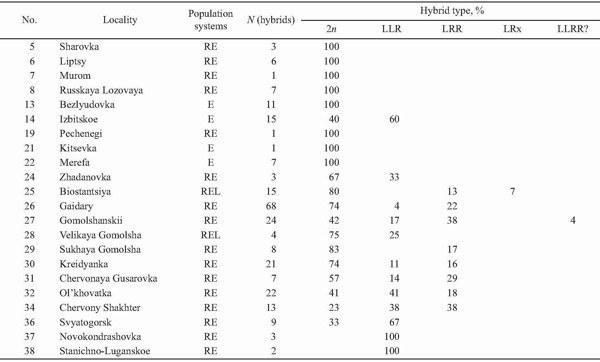

Fig. 10. A floodplain of Seversky Donets River near Biostantsiya. The locality was inhabited by Rana lessonae (occasional), R. ridibunda, and R. esculenta. Hybrids were represented by both diploids and triploids (LLR and LRR). Photo by Dmitry A. Shabanov.

Fig. 11. A floodplain of Seversky Donets River near the village Kreidyanka, Kharkov Province. The locality was inhabited by Rana ridibunda and R. esculenta. Hybrids were represented by both diploids and triploids (LLR and LRR). Photo by Dmitry A. Shabanov.
DISCUSSION
Historical Background
Based on his field observations in years 1848 and 1849, Alexander Czernay (1852), Extraordinary Professor of Kharkov University, classified all green frogs, distributed in Kharkov Government, to the same species R. viridis Roesel, with three so called varieties.
The eminent Russian herpetologist Alexander Nikolsky, Professor of Kharkov University (since 1903) also applied the single-species concept to European green frogs (with three subspecies). He suggested that European part of Russian Empire, including Kharkov Government was inhabited by R. esculenta ridibunda only (Nikolsky, 1918).
Pavel Terentjev (1927) introduced two-species scheme, with R. ridibunda and R. esculenta, each species was splitted in two subspecies. Based on St. Petersburg (Leningrad) and Moscow collections, he listed the former species for Zmiyov, Kharkov Province (“Zmiersk, gub. Kharkow”). According to P. V. Terentjev, European part of the Soviet Union was inhabited by R. ridibunda ridibunda and R. esculenta lessonae. This scheme was widely accepted by Soviet zoologists after the publication of the classic guide book on the herpetofauna of the USSR co-authored by P. V. Terentjev and S. A. Chernov (1949).
Valery Vedmederja (1984) from the Museum of Nature, Kharkov State University, was able to distinguish three species of green frogs occurred in Kharkov Province. He applied morphological and ecological (habitat) approaches to identify these species. Rana lessonae preferred to inhabit various forest stagnant water bodies in Krasnokutsk District (Vladimirskoe Forestry, a “pure” population), as well as in Zachepilovka, Kharkov, and Volchansk Districts in the northwest, southwest and northeast of Kharkov Province. In the latter two districts, the species was recorded with R. esculenta together, often in the same habitat. The lake frog, R. ridibunda was distributed in many districts of the province, except the northern Volchansk, Krasnokutsk, and Bogodukhov Districts. As a rule, the species liked running water bodies. The hybrid R. esculenta inhabited both types of water bodies, coexisting with R. ridibunda or R. lessonae. All three species occupied “Orchik” (= Russkii Orchik) site in Zachepilovka District. However, Iskov Pond near Gaidary village was settled by R. esculenta only. Vedmederya (1984) also mentioned that this species was represented by two morphological forms with lessonae-like and ridibunda-like frogs. The both forms can occur in the same water bodies, often with R. ridibunda together.
More detailed historical sketch of green frog studies, in the 20th century in particular, has been published by Korshunov et al. (2004).
In 1983 – 1994, we studied green frogs from the vast territory of the Central Chernozem (“black soil” in Russian) Region of Russia, including Kursk, Belgorod, and Voronezh Provinces which bordered with eastern Ukraine (Lada et al., 1995). To extent that research geographically, in 1995 and 1996, we (G. A. Lada) collected two samples from the large Iskov Pond and from Koryakov Yar situated in Zmiyov District (Lada, 1998, 1999). The points “Orchik” in Zachepilovka District, and “Ol’khovaya balka” in Kharkov District were visited as well. These field trips to Kharkov Province were stimulated by V. I. Vedmederja’s published data. In 2002 our current cooperative project started.
Polyploidy
1. Geographic distribution of triploidy
Some authors mentioned the strange geographic distribution of triploidy in wild populations of Rana esculenta. Such an enigmatic situation is without serious explanation. Indeed, triploids were often revealed in many localities of northern France, the Netherlands, northern Germany, Denmark, southern Sweden, Poland, western Slovakia, and western Hungary (Table 5). However, triploids are virtually absent in surveyed areas of Austria, Italy, ex-Yugoslavia, and the former Soviet Union (Graf and Polls Pelaz, 1989a). Triploids are abundant especially in the northwestern part of the range of R. esculenta, while they are less frequent in other European regions. It seems that triploids are totally absent from eastern Europe, the Italian and Balkan peninsulas (Mikulí è ek and Kotlík, 2001). Obviously, triploids are common in the western Baltic area.
Curiously, five triploids, or 1.5%, out of 336 R. lessonae frogs were reported from the Netherlands (Blommers-Schlösser, 1990). Later, Okulova et al. (1997) found two triploid animals, or 4.3%, of this species in Ivanovo Province, central European Russia (n = 47). Both cases were based on erythrocyte size only. However, verification is needed by karyotyping or DNA cytometry. Triploidy was reported for North African green frogs (R. saharica) as well (Hemmer et al., 1980). Moreover, this phenomenon was found in R. grafi, another hybrid form in western Europe (France) arosen from the hybridization between R. perezi and R. ridibunda (Schmeller et al., 2001).
Three triploid R. esculenta identified by the albumin electrophoresis were reported from Lower Austria; the sample size is unknown (Schneider and Tunner, 1982). Occasional frogs with triploid erythrocyte size were found in Bohemia and Moravia, Czech Republik (Souè ek et al., 1993; Zavadil, 1994; Zavadil et al., 1999). Two obviously occasional triploid or tetraploid specimens confirmed by genome size and chromosome count, respectively, were also recorded from the eastern Baltic area, Kaliningrad Province of Russia and Latvia, respectively (Borkin et al., 1979; our unpublished data). Using erythrocyte size, Okulova et al. (1997) suggested occasional triploidy in R. esculenta (single specimen) from Ivanovo Province, central European Russia, although our DNA flow cytometric analysis did not registered any triploidy among Ivanovo frogs. Apart from these data, no polyploid green frogs have yet been reported from enormous European territory of the former Soviet Union (Mazin and Borkin, 1979; Borkin et al., 1987; Vinogradov et al., 1988, 1990, 1991; Caune and Borkin, 1993; Lada et al., 1995; Borkin et al., 2003a, 2003b). Several studies, which were focused on green frog populations of Ukraine, showed no triploidy (Mazin and Borkin, 1979; Mezhzherin and Morozov-Leonov, 1992, 1993, 1996; Morozov-Leonov and Mezhzherin, 1995; Lada, 1999; our unpublished data).
In his PhD dissertation abstract, Morozov-Leonov (1998) provided a brief mention that six out of 25 hybrid frogs from Ukrainian Transcarpathians displayed the gene-dose effect in albumin, which was suggested to be an evidence for triploidy (“allotriploidy”). Unfortunately, no other data or comments about the case were given. In recent paper (Morozov-Leonov et al., 2003) devoted to hybrids of the same region, the karyotype analysis indicated diploid level of all hybrids examined. We failed to reveal any triploidy among R. esculenta from Ukrainian Transcarpathians (our unpublished data).
Rybacki and Berger (2001) provided an important analysis of the distribution of triploids in Poland. The authors mentioned that the picture seen in the western and eastern parts of the country is similar to the distribution patterns found in the corresponding neighbouring areas. In Poland the highest triploid proportion was observed in the northwestern districts Szczecin and Gdañsk (23% and 21% among all water frogs examined, respectively). This region is close to northeastern Germany where triploids are especially numerous (up to 90%). In southern Poland, as in the Czech Republic and Slovakia, triploids are usually rare. In this respect the only exception was the Wroc³aw District (whith 11% of triploids). In the eastern districts of Poland, only a few triploids were found, and in the territory of the former Soviet Union triploid R. esculenta seems to be almost absent. However, Schröer (1996) found that 45% out of 50 individuals examined from eastern Poland, Bialystok District, bordered with Byelorussia, proved to be triploids. We found no triploids in Byelorussia (Borkin et al., 1986; our unpublished data).
Thus, our finding of mass occurrence of triploids in Ukraine is quite surprising. This should be regarded as the first reliable record of triploid R. esculenta in eastern Europe in general, and in eastern Ukraine in particular. It is worth noting that these triploids are isolated from the nearest 3n localities approximately by the distances of 1000 (eastern Poland) and 1500 km (western Hungary).
Apart from numerous triploids, a tetraploid hybrid was revealed by us in 2003 in Verkhnii Gomolshanskii pond, Zmiyov District, Kharkov Province (Figs. 2 and 4; Tables 2 and 3). This throws new light on previous tetraploid record from the environs of Riga, Latvia, where a R. esculenta male with four chromosome sets was found among 32 animals (Borkin et al., 1979). Both localities are separated by a distance of about 1120 km. No case of natural tetraploidy in R. esculenta is known in other parts of Europe.
TABLE 5. Geographical Distribution of Triploidy in Natural Populations of European Hybrid Green Frogs Rana esculenta and R. grafi, and Methods of Its Identification (occasional occurrences of triploids are not included)

2. Methods of polyploidy identification
Various authors applied different techniques to check ploidy level in green frog samples (Table 5). To evaluate the reliability and importance of the published data, we give the brief comparison of methods.
Chromosome number. The chromosome counting, of course, provides direct evidence for any kind of polyploidy (e.g., Günther, 1970). However, this method would be quite labour-intensive to examine numerous samples containing tens or hundreds of animals.
Erythrocyte size. Since the mid 1970es, the cell size has been proposed as a useful tool for ploidy determination in green frogs. That cell size varies with ploidy level in amphibians has long been known. The difference is particularly marked in red blood cells (erythrocytes), and the size of these cells has been used previously to distinguish diploid and triploid ambystomes (Uzzell, 1963) and green frogs (Günther, 1975a; Uzzell and Berger, 1975). The positive correlation between erythrocyte size and ploidy level in green frogs has been confirmed by several authors because cell size data were in correspondence with the chromosome number, protein electrophoresis or nuclear DNA cytometry data (Uzzell et al., 1975; Günther, 1977; Ogielska-Nowak, 1978; Polls Pelaz and Graf, 1988; Plötner and Klinkhardt, 1992). Moreover, the cell size technique allowed to make air dried blood smears for tadpoles and frogs of various age, including postmetamorphosed froglets, by removing tips of tails or digits, respectively. Importantly, such an approach allows working with animals in the field and laboratory, without killing them. Therefore, it was no wonder that this very easy and cheap tool has been widely accepted as a good indicator of ploidy level, and the majority of triploidy records was evidenced by erythrocye size only (Table 5).
However, in young animals the difference in blood cells size between diploids and triploids was not so distinct (Ogielska-Nowak, 1978). Sometimes, different conditions during the cell drying process might result in cell parameters (Plötner and Klinkhardt, 1992). Later, Schröer (1996) argued that the comparison of erythrocyte size was inadequate to identify different ploidy level. Ogielska et al. (2001) found that the correlation between erythrocyte area and DNA content was quite high (r = 0.82), however, large cells may have low DNA content, and vice versa. The authors concluded that the erythrocyte size is not always a good indicator of ploidy. Finally, results presented by Schmeller et al. (2001) supported significant influence of several factors (like body length, geographic location, organic matter of substratum, and relative amount of oxygen) on the erythrocyte size in hybrids. Even within single populations up to 16.7% of individuals may be wrongly affiliated to ploidy level. These authors agreed with Schröer’s criticism that the cell planimetry is less suitable for ploidy determination. Taking into considerations such recent conclusions, we should use the literature data based on cell size only with some precaution. It is worth noting that cell size does not enable to distinguish triploids with different genome composition, LLR and LRR, respectively.
Morphometric indices. Some experienced authors used the external morphological characters expressed, for instance, by DP/CI and T/CI indices to distinguish diploid and triploid hybrids as well as both kinds of triploids because genome-dosage effects are seen in morphological features (e.g., Berger et al., 1978; Berger and Truszkowski, 1980; Fog, 1995; Rybacki and Berger, 2001). Indeed, for instance, Berger and Günther (1988) demonstrated that individuals with the same genome composition had similar indices in every study population, and those with different genome composition showed clearly marked differences. However, formerly, Günther (1975a, p. 157) stated that “among the triploid edible frogs there was a greater morphological overlap towards lessonae and ridibunda than among the diploids, although most of the 3n individuals could be included within the range of variability of the 2n animals.” Later, Plötner and Klinkhardt (1992) postulated that a clear classification of triploids into LLR and LRR genotypes is not possible, and only slight differences exist between triploid and diploid R. esculenta. In fact, in several studies, morphological identification did not correspond with electrophoretic data, and geographic variation in external traits has been suggested (e.g., Hemmer, 1977; Regnier and Neveu, 1986; Günther et al., 1991). Rybacki (1995a, 1995b) provided the ranges of DP/CI and T/CI indices, with obvious overlap between diploid and triploid R. esculenta, as well as triploids with R. ridibunda (for LR and LLR also see Table 5 in Berger and Truszkowski, 1980).
According to Schröer (1997), the results of this method varied strongly in different populations, and increased overlap between R. lessonae, diploid and triploid R. esculenta was observed (Schröer, 1996). In contrast to progeny of triploids from Poland, marked overlap in morphometric characters (DP/CI against T/CI) between diploid hybrids and their parental species was evidenced for that from eastern Germany (Günther et al., 1979). Pagano and Joly (1999), based on specimens identified by allozyme analysis, found that morphological indices in R. esculenta and R. ridibunda widely overlapped, and most of the hybrids could not be distinguished from R. ridibunda using these indices. They asserted that morphometric identification might be far from being secure. In our studies performed by means of DNA flow cytometry, we also revealed that the ranges of indices in diploid R. esculenta and their parental species may overlap, and the identification of some individuals by morphometric characters may be confused (Lada et al., 1995; Borissovsky et al., 2000; Nekrasova and Morozov-Leonov, 2001).
It is widely accepted that, according to the gene-dosage effect, the LLR esculenta should be more similar to R. lessonae, while for LRR frogs, in contrary, a higher morphometric similarity to R. ridibunda can be expected. However, for triploids from some East German populations, this rule was contested (Plötner et al., 1994).
Protein electrophoresis. Some authors checked ploidy level in hybrid green frogs used the electrophoretical data. In accordance with the morphological traits, gene-dosage effects visible in more heavily stained albumin (or other proteins) bands indicate that such specimens were triploid. The technique also allows to recognize the genome composition in triploids, LLR or LRR. Curiously, a specimen of R. esculenta with diploid erythrocyte size displayed the triploid effect in the lactate dehydrogenase locus LDH-B (Günther and Hähnel, 1976), while several frogs with larger cells were diploid (Gubányi and Korsós, 1992). However, obvious genedosage effects in some triploids may be not revealed (Ebendal and Uzzell, 1982; Plötner and Klinkhardt, 1992; Mikulí è ek and Kotlík, 2001; see also Schröer, 1996). This gene dosage effect can be demonstrated by cellogel or polyacrylamide gel electrophoresis, but is not always clear with strach gel electrophoresis (Hemmer et al., 1980). This technique is quite labor-intensive to treat many large samples, and sometimes, the data interpretation may be not simple. Only few authors applied the method to recognize triploidy (e.g., Blommers-Schlösser, 1990; Tunner and Heppich-Tunner, 1992).
Genome size. The first papers based on the nuclear DNA content (genome size) in green frogs were published in late 1970s (Tunner and Dobrowsky, 1976; Ogielska-Nowak, 1978; Mazin and Borkin, 1979). Several cytophotometric techniques exist, like microdensitometry with staining nuclei by Feulgen method (Ogielska-Nowak, 1978; Hemmer et al., 1980; Konrad et al., 1980) or microfluorimetry with use of fluorescent Schiff reagent (Mazin and Borkin, 1979; Ogielska et al., 2001), DNA flow cytometry (our studies since 1985). Currently, this is the most reliable method which can provide relatively quickly the next data set about each specimen: 1) species identification, 2) ploidy level, 3) genome composition in hybrids, 4) type of gametes (spermatozoa) in terms of ploidy and genome composition. The application of this method can allow to solve various problems of population genetics, gametogenesis, etc., associated with the R. esculenta complex. However, the laboratory equipment (the cytophotometer with computers) is quite expensive, and the work needs a good experience. The future progress of the approach will be associated with the development of cheaper portable cytophotometer and taking blood cells without animal killing.
Bioacoustic approach. Some differences in mating call were found between diploid and triploid R. esculenta from Austria; triploidy was evidenced from plasma albumin staining intensity (Schneider and Tunner, 1982).
Some conclusion can be drawn from these brief comments. External morphology, erythrocyte size, and protein electrophoresis, certainly, can indicate the existence of triploidy among green frog populations. However, real data, based on indices or cell size in particular, may be biased. In fact, karyotype and genome size analyses are more appropriate for exact ploidy determination (Schmeller et al., 2001). The application of DNA flow cytometry, being the most precise technique, may provide new opportunities for the study on green frogs, especially in connection with other methods. Frankly speaking, any choice of approach should be adequate to the problem, which should be solved.
We agreed with Ogielska et al. (2001) that the problem of ploidy is more complicated than it was assumed, and we cannot rule out the existence of aneuploids or mosaics among the hybrids (Berger and Ogielska, 1994). Another serious problem is the instability or irregularities in clonal inheritance mechanisms, which, probably, occur more frequently in populations with triploids (Günther, 1975b; Uzzell et al., 1977; Vinogradov et al., 1990).
3. Mass occurrence of triploidy in eastern Ukraine
Based mainly on erythrocyte size measurements, Günther (1975a) calculated that in the northeastern part of Germany, the share of triploids varied between 15% and 83% among all hybrids (2n + 3n together) in populations. As a whole, the percentage of triploids was equal to about 39% out of R. esculenta (which was examined in the region). Numerous triploids (62 – 100%, recalculated by us) were identified by Berger and Günther (1988) among hybrids collected near Serrahn, Mecklenburg, with sample size between 6 and 88 individuals (100% hybrid occurrence was registered in the smallest sample). Using various techniques, including chromosome counting, Plötner and Klinkhardt (1992) found that triploidy reached about 61% among 46 frogs taken from artificial forest pools in northern Germany as well. Hemmer (1977) and Eikhorst (1981) reported that triploids were very common in some localities in northwestern Germany (between 21.5% and 83%).
In a pure R. esculenta population existed in the south of the Netherlands, triploids were about 64% (Blommers-Schlösser, 1990). In France triploid hybrids of the LLR composition reached 19% in a population with R. lessonae (Polls Pelaz and Graf, 1989).
In central Europe, the frequency of triploid R. esculenta obviously decreases from northern Germany to the south and to the east (Rybacki and Berger, 2001:Fig. 2). For instance, in the westernmost part of Slovalia, 3n frogs composed 4 – 12% out of hybrids (recalculated by us from Table 1; Mikulí è ek and Kotlík, 2001). High incidence of triploids in northeast Poland (58 – 84%) reported by Schröer (1996) was disputed by Rybacki and Berger (2001). However, in western Hungary, 50 triploids, or 96%, were detected electrophoretically and karyologically among 52 hybrids (Tunner and HeppichTunner, 1992).
In eastern Ukraine, among all hybrids, the share of triploid individuals was equal to 36% (Table 2), ranging from 20% to 76% in localities (Table 4). In two small samples (Novokondrashovka and Stanichno-Luganskoe, with 3 and 2 hybrids, respectively), they composed 100%. Therefore, the triploid portion in east-Ukrainian populations was similar to that in northern Germany or Hungary. Thus, the trend of decreasing occurrence of triploids did not correct for eastern Ukraine. Nevertheless, such a mass occurrence in the region is quite surprising because we failed to reveal the triplody occurrence in adjacent territories of Russia despite of our extensive screening frog populations in the Central Chernozem Territory and in Volga River Basin (Lada et al., 1995; Borissovsky et al., 2000, 2001; Borkin et al., 2003a, 2003b).
4. Triploidy and population systems
In his remarkable paper about the triploidy, Günther (1975a) reported on the occurrence of triploid hybrids in several types of population systems. These were socalled “pure” E type consisting of R. esculenta only, i.e., without any parental species, the L-E type (e-l and l-e of Günther), where hybrids co-existed with R. lessonae, as well as the R-E type (e-r) consisting of hybrids and R. ridibunda.
It should be noted that Günther (1975a) proposed a detailed classification of population systems, which reflected the genome structure, sex ratio, and relative frequencies of species in mixed populations. In contrast to this paper, we use more simplified designation of population systems based on the species existence only. In these systems, the portion of triploids was equal to 20 – 83% (E), 15 – 43% (L-E), and 41% (R-E).
Two other localities with high share of triploids in northern Germany were occupied by the pure E type of population systems (Eikhorst, 1981; Plötner and Klinkhardt, 1992). However, in Hungary high incidence of triploidy was associated with the R-E type (Tunner and Heppich-Tunner, 1992).
In France (Polls Pelaz and Graf, 1989), the RRL triploidy was identified in the L-E type. In Poland (Rybacki and Berger, 2001, Table 2), triploids are known from various kinds of the E, L-E, and R-E types, as well as, rarely, in the L-E-R type of population systems.
In eastern Ukraine, triploids were revealed in three types of population systems. These were the E type (60%), the R-E type (27 – 76%, and 100% in two samples of small size), as well as the L-E-R type (17 – 55%). Thus, unlike in northern Germany, triploids under the study did not reach the highest frequency in pure hybrid populations. Moreover, triploids were recorded from the mixed population system of the L-E-R type, where all members of the R. esculenta complex exist together. Interestingly, this complicated system is very rare in western and central Europe. However, the system is widely distributed in the Central Chernozem Territory and Volga River Basin in Russia (Lada et al., 1995; Borissovsky et al., 2001; Borkin et al., 2003a). Nevertheless, unlike in Ukraine, we failed to find any triploids in these regions.
Acknowledgments. We thank T. V. Babinich (Kharkov), V. A. Dyakov (Svyatogorsk), S. S. Fomenko (Kharkov), T. S. Fomenko (Balakleya), V. P. Foroshchuk (Lugansk), L. A. Goncharenko (Kharkov), T. N. Goncharenko (Kharkov), M. A. Kravchenko (Kharkov), and A. M. Rudik (Kharkov; now San Francisco, USA) for providing materials and for the field assistance as well as A. E. Vinogradov (St. Petersburg) for some genome size data (1989 – 1996). This work was supported by the Russian Foundation for Basic Research (grants Nos. 02-04-49631 and 04-04-63165), by the Russian Federal State Program “Integration” (grants Nos. E-0121 and E-3248), and by the Russian Federation President’s grant for the support of scientific schools (NSh. 1647.2003.4).
REFERENCES
Anonym (1992), Topograficheskaya Karta. Khar’kovskaya Oblast’. Masshtab 1:300000 [Topographical Map. Kharkov Province. Scale 1:300,000], TU MO Ukrainy [in Russian].
Berger L. (1988), “An all-hybrid water frog population persisting in agrocenoses of central Poland (Amphibia, Salientia, Ranidae),” Proc. Acad. Natur. Sci. Philadelphia, 140(1), 202 – 219.
Berger L. (1990 [1988]), On the origin of genetic systems in European water frog hybrids,” Zool. Polon., 35(1 – 4), 5 – 32.
Berger L. and Berger W. A. (1994), “Persistence of all-hybrid water frog populations (Rana kl. esculenta) in northern Germany,” Genet. Polon., 35(1 – 2), 73 – 80.
Berger L. and Günther R. (1988), “Genetic composition and reproduction of water frog populations (Rana kl. esculenta Synklepton) near nature reserve Serrahn, GDR,” Arch. Naturschutz Landschaftsforsch., 28(4), 265 – 280.
Berger L. and Günther R. (1991 – 1992), “Inheritance patterns of water frog males from the environments of Nature Reserve Steckby, Germany,” Zool. Polon., 37(1 – 4), 87 – 100.
Berger L. and Ogielska M. (1994), “Spontaneous haploidtriploid mosaicism in the progeny of Rana esculenta female and Rana lessonae male,” Amphibia–Reptilia, 15(2), 143 – 152.
Berger L., Roguski H., and Uzzell T. (1978), “Triploid F2 progeny of water frogs (Rana esculenta complex),” Folia Biol., 26(3), 135 – 152.
Berger L. and Truszkowski J. (1980), “Viability and inheritance of characters in water frogs (Rana esculenta complex) in agrocenozes,” Genet. Polon., 21(3), 309 – 323.
Blommers-Schlösser R. M. A. (1990), “On the occurrence and identity of triploids of Rana kl. esculenta Linnaeus and Rana lessonae Camerano in the Netherlands (Anura: Ranidae),” Bijdr. Dierk., 60(3/4), 199 – 207.
Borissovsky A. G., Borkin L. J., Litvinchuk S. N., and Rosanov J. M. (2000), “Morphometric characterization of green frogs (Rana esculenta complex) in Udmurtia,” Vestnik Udmurt. Univ. [in Russian], 5, 70 – 75.
Borissovsky A. G., Borkin L. J., Litvinchuk S. N., and Rosanov J. M. (2001), “The distribution of green frogs (Rana esculenta complex) in Udmurtia,” Vestnik Udmurt. Univ. [in Russian], 5, 51 – 63.
Borkin L. J. (2001), “Speciation, hybridization, and polyploidy in amphibians of the Palearctic,” in: The Problems of Herpetology [in Russian], Pushchino – Moscow, pp. 46 – 48.
Borkin L. J., Caune I. A., Pikulik M. M., and Sokolova T. M. (1986), “Distribution and structure of the green frog complex in the USSR,” in: Z. Ro è ek (ed.), Studies in Herpetology, Prague, pp. 675 – 678.
Borkin L. J., Garanin W. I., Tichenko N. D., and Zaune I. A. (1979), “Some results in the green frog survey in the USSR,” Mitt. Zool. Mus. Berlin, 55(1), 153 – 170.
Borkin L. J., Litvinchuk S. N., Mannapova E. I., Pestov M. V., and Rosanov J. M. (2003a [2002]), “The distribution of green frogs (Rana esculenta complex) in Nizhny Novgorod Province, central European Russia,” Russ. J. Herpetol., 9(3), 195 – 208.
Borkin L. J., Litvinchuk S. N., Rosanov J. M., Lada G. A., Ruchin A. B., Faizulin A. I., and Zamaletdinov R. I. (2003b), “The hybridogenetic Rana esculenta complex: does “the Volga paradox” exist?,” in: Proc. of the Third Conf. of Herpetologists of Volga Region [in Russian], Tol’yatti, pp. 7 – 12.
Borkin L. J., Litvinchuk S. N., Rosanov J. M., and Milto K. D. (2001), “Cryptic speciation in Pelobates fuscus (Anura, Pelobatidae): evidence from DNA flow cytometry,” Amphibia–Reptilia, 22(4), 387 – 396.
Borkin L. J., Vinogradov A. E., Rosanov J. M., and Caune I. A. (1987), “Hemiclonal inheritance in the hybridogenetic complex of Rana esculenta: evidence from DNA flow cytometry,” Dokl. Akad. Nauk SSSR [in Russian], 295(5), 1261 – 1264.
Bucci S., Ragghianti M., Mancino G., Berger L., Hotz H., and Uzzell T. (1990), “Lampbrush and mitotic chromosomes of the hemiclonally reproducing hybrid Rana esculenta and its parental species,” J. Exp. Zool., 255(1), 37 – 56.
Caune I. A. and Borkin L. J. (1993), “A new kind of unisexual-bisexual population systems in European green frogs (Rana esculenta complex),” in: E. N. Panov and P. S. Tomkovich (eds.), The Hybridization and Species Problem in Vertebrates. Arch. Zool. Mus. Moscow State Univ. Vol. 30 [in Russian], Moscow, pp. 34 – 52.
Czernay A. (1852), Fauna of Kharkov Government and Adjacent Territories. Part 1. Fauna of Amphibian Animals and Fishes [in Russian], Izd. Kharkov Univ., Kharkov.
Dubois A. (1990), “Nomenclature of parthenogenetic, gynogenetic and “hybridogenetic” vertebrate taxons: new proposals,” Alytes, 8(3 – 4), 61 – 74.
Dubois A. and Günther R. (1982), “Klepton and synklepton: two new evolutionary systematics categories in zoology,” Zool. Jb. Syst., 109(2), 290 – 305.
Ebendal T. and Uzzell T. (1982), “Ploidy and immunological distance in Swedish water frogs (Rana esculenta complex),” Amphibia–Reptilia, 3(2/3), 125 – 133.
Eikhorst R. (1981), “Populationsgenetische Untersuchungen an Grünfröschen der Bremer Umgebung,” Beitr. Naturk. Niedersachsens, 34, 104 – 111.
Eikhorst R. (1988), “Die Verbreitung von diploiden und triploiden Larven des Teichfrosches Rana esculenta Linnaeus, 1758 in einer reinen Bastardpopulation,” Salamandra, 24(1), 59 – 68.
Fog K. (1995 [1994]), “Water frogs in Denmark: population types and biology,” Zool. Polon., 39(3/4), 305 – 330.
Graf J.-D. and Polls Pelaz M. (1989a), “Evolutionary genetics of the Rana esculenta complex,” in: R. M. Dawley and J. P. Bogart (eds.), Evolution and Ecology of Unisexual Vertebrates, New York State Mus., Albany, New York, Bull. 466, pp. 289 – 302.
Graf J.-D. and Polls Pelaz M. (1989b), “Cytogenetic analysis of spermatogenesis in unisexual allotriploid males from a Rana lessonae — Rana kl. esculenta mixed population,” in: T. Halliday, J. Baker, and L. Hosie (eds.), Abstrs. of the First Congr. of Herpetol., September 11 – 19, 1989. University of Kent at Canterbury, UK, University of Kent, Canterbury [without pagination].
Gubányi A. and Korsós Z. (1992), “Ploidy of Rana esculenta specimens of an E-L popualation in the Kis-Balaton landscape protection area,” in: Z. Korsós and I. Kiss (eds.), Proc. Sixth Ord. Gen. Meet. Soc. Eur. Herpetol. 1991. Hungar. Natur. Hist. Mus., Budapest, pp. 215 – 218.
Günther R. (1970), “Der Karyotyp von Rana ridibunda Pall. und das Vorkommen von Triploidie bei Rana esculenta L. (Anura, Amphibia),” Biol. Zentralblatt, 89(3), 327 – 342.
Günther R. (1975a), “Zum natürlichen Vorkommen und zur Morphologie triploider Teichfrösche, “Rana esculenta,” L., in der DDR (Anura, Ranidae),” Mitt. Zool. Mus. Berlin, 51(1), 146 – 158.
Günther R. (1975b), “Untersuchungen der Meiose bei Männchen von Rana ridibunda Pall., Rana lessonae Cam. und der Bastardform “Rana esculenta” L. (Anura),” Biol. Zentralblatt, 94(3), 277 – 294.
Günther R. (1977), “Die Erythrozytengröße als Kriterium zum Unterscheidung diploider und triploider Teichfrösche, Rana “esculenta” L. (Anura),” Biol. Zentralblatt, 96(4), 457 – 466.
Günther R. (1990), Die Wasserfrösche Europas (Anura — Froschlurche), A. Ziemsen, Wittenberg – Lutherstadt (Die Neue-Brehm-Bücherei, 600).
Günther R. (1991), “Europäische Wasserfrösche (Anura, Ranidae) und biologisches Artkonzept,” Mitt. Zool. Mus. Berlin, 67(1), 39 – 53.
Günther R. (1997), “Rana kl. esculenta Linnaeus, 1758,” in: J.-P. Gasc et al. (eds.), Atlas of Amphibians and Reptiles in Europe. Societas Europaea Herpetologica and Muséum National d’Histoire Naturelle, Paris, pp. 138 – 139.
Günther R. and Hähnel S. (1976), “Untersuchungen über den Genfluß zwischen Rana ridibunda und Rana lessonae sowie die Rekombinationsrate bei der Bastardform Rana “esculenta” (Anura, Ranidae),” Zool. Anz., 197(1/2), 23 – 38.
Günther R. and Plötner J. (1989 – 1990), “Mating pattern in pure hybrid populations of water frogs, Rana kl. esculenta (Anura, Ranidae),” Alytes, 8(3 – 4), 90 – 98.
Günther R., Plötner J., and Tetzlaff I. (1991), “Zu einigen Merkmalen der Wasserfrösche (Rana synkl. esculenta) des Donau-Deltas,” Salamandra, 27(4), 246 – 265.
Günther R., Uzzell T., and Berger L. (1979), “Inheritance pattern in triploid Rana “esculenta” (Amphibia, Salientia),” Mitt. Zool. Mus. Berlin, 55(1), 35 – 57.
Hemmer H. (1977), “Studien an einer nordwestdeutschen Grünfroschpopulation als Beitrag zur Bestimmungsproblematik und zur Rolle der Selektion im Rana esculenta Komplex (Amphibia: Salientia: Ranidae),” Salamandra, 13(3/4), 166 – 173.
Hemmer H., Konrad A., and Bachmann K. (1980), “Hybridization within the Rana ridibunda complex in North Africa,” Amphibia–Reptilia, 1(1), 41 – 48.
Hotz H., Beerli P., and Spolsky C. (1992), “Mitochondrial DNA reveals formation of nonhybrid frogs by natural matings betwen hemiclonal hybrids,” Mol. Biol. Evol., 9(4), 610 – 620.
Knudsen K. and Scheel J. J. (1975), “Contribution to the systematics of Europaean green frogs,” Bull. Soc. Zool. France, 100(4), 677 – 679.
Konrad A., Bachmann K., and Hemmer H. (1980), “Erythrocytenkern-DNA-Bestimmungen bei Rana perezi im Rahmen des paläarktischen Grünfroschkomplexes (Amphibia: Salientia: Ranidae),” Salamandra, 16(1), 57 – 59.
Korshunov A. V., Babinich T. V., Zinenko A. I., and Shabanov D. A. (2004), “Diversity of green frogs (Rana esculenta complex) in the Kharkov Province: morphological aspect of studying,” Biol. Valeol. [in Ukrainian], Kharkov, 6, 24 – 30.
Lada G. A. (1995), “Middle European green frogs (the hybridogenetic Rana esculenta complex): an introduction to the problem,” in: V. M. Kolontaev (ed.), Flora and Fauna of the Chernozem Territory [in Russian], Izd. TGU, Tambov, pp. 88 – 109.
Lada G. A. (1998), “On the necessity of the protection of unique “pure” populations of diploid edible frog (Rana esculenta Linnaeus, 1758) in Belgorod and Kharkov Provinces,” in: Problemy Okhrany i Ratsionalnogo Ispol’zovaniya Prirodnykh Ecosystem i Biologicheskikh Resursov (Problems of Protection and Rational Use of Natural Ecosystems and Biological Resources) [in Russian], Penza, pp. 333 – 335.
Lada G. A. (1999), “Pure diploid hybridogenetic populations of R. kl. esculenta (Linnaeus, 1758) in Central Russia and Eastern Ukraine,” in: D. Schmeller and J. Plötner (eds.), Abstrs. of the III Int. Symp. “Genetics, Systematics and Ecology of Western Palearctic Water Frogs,” October 11 – 15, 1999, Johannes Gutenberg Universität Mainz, Mainz, p. 11.
Lada G. A., Borkin L. J., and Vinogradov A. E. (1995), “Distribution, population systems and reproductive behavior of green frogs (hybridogenetic Rana esculenta complex) in the Central Chernozem Territory of Russia,” Russ. J. Herpetol., 2(1), 46 – 57.
Mazin A. L. and Borkin L. J. (1979), “Nuclear DNA content in green frogs of the genus Rana,” Mitt. Zool. Mus. Berlin, 55(1), 217 – 224.
Mezhzherin S. V. and Morozov-Leonov S. J. (1992), “Aberrant phenotypes of Ldh-B locus in hybrid populations of the Rana esculenta complex (Amphibia, Ranidae),” Dokl. Akad. Nauk SSSR [in Russian], 324(6), 1314 – 1317.
Mezhzherin S. V. and Morozov-Leonov S. J. (1993), “Population genetic analysis of the structure of hybrid populations of the Rana esculenta L. complex (Amphibia, Ranidae),” Tsitol. Genet. [in Russian], 27(2), 63 – 68.
Mezhzherin S. V. and Morozov-Leonov S. J. (1996), “Genetic analysis of the structure of hybrid populations of green frogs, the Rana esculenta L. complex (Amphibia, Ranidae), from Volyñ,” Tsitol. Genet. [in Russian], 30(1), 48 – 53.
Mikulíèek P. and Kotlík P. (2001), “Two water frog populations from western Slovakia consisting of diploid females and diploid and triploid males of the hybridogenetic hybrid Rana esculenta (Anura, Ranidae),” Mitt. Mus. Naturkunde Berlin. Zool. Reihe, 77(1), 59 – 64.
Morozov-Leonov S. J. (1998), Genetic Processes in Hybrid Populations of Green Frogs, the Rana esculenta L. Complex in Ukraine. Author’s Abstratct of Candidate’s Thesis [in Ukrainian], Kiev.
Morozov-Leonov S. J. and Mezhzherin S. V. (1995), “An analysis of genetic structure of the hybrid population of green frogs, the Rana esculenta complex, from the Danubian plavni,” Tsitol. Genet. [in Russian], 29(2), 71 – 76.
Morozov-Leonov S. J., Mezhzherin S. V., and Kurtyak T. T. (2003), “The genetic structure of the unisex hybrid Rana esculenta complex populations in the Transcarpathians Lowland,” Tsitol. Genet. [in Russian], 37(1), 43 – 47.
Nekrasova O. D. and Morozov-Leonov S. Y. (2001), “Diagnostics of frogs of the Rana esculenta complex (Amphibia, Ranidae) in hybrid populations in the Dnieper River area,” Vestn. Zool. [in Russian], 35(5), 45 – 50.
Nikolsky A. M. (1918), Amphibia [in Russian], Russ. Acad. Sci., Petrograd.
Ogielska-Nowak M. (1978), DNA content in erythrocyte nuclei of diploid and triploid green frog hybrid of Rana esculenta L. complex,” Zool. Polon., 27(1), 109 – 115.
Ogielska M., Kubicius I., Gruszka K., Odziomek B., Porêba M., Indyk M., Kazana K., and Ferenc M. (1995 [1994]), “Diploid-triploid, predominantly male pure Rana esculenta population in Wroc³aw — Nowy Dom,” Zool. Polon., 39(3 – 4), 5017 – 502.
Ogielska M., Kazana K., and Kierzkowski P. (2001), “DNA content in erythrocyte nuclei of water frogs from a pure Rana esculenta population in Dêbki (Gda ñ sk district, Poland),” Mitt. Mus. Naturkunde Berlin. Zool. Reihe, 77(1), 65 – 70.
Okulova N. M., Borkin L. J., Bogdanov A. S., and Guseva A. (1997), “The green frogs in Ivanovo Province,” Adv. Amphibian Res. Former Soviet Union, 2, 71 – 94.
Pagano A. and Joly P. (1999), “Limits of the morphometric method for field identification of water frogs,” Alytes, 16(3 – 4), 130 – 138.
Plötner J. and Klinkhardt M. (1992), “Investigations on the genetic structure and the morphometry of a pure hybrid population of Rana kl. esculenta (Anura, Ranidae) in North Germany,” Zool. Anz., 229(3/4), 163 – 184.
Plötner J., Becker C., and Plötner K. (1994), “Morphometric and DNA investigations into European water frogs (Rana esculenta Synklepton (Anura, Ranidae)) from different population systems,” J. Zool. Syst. Evol. Res., 32(3), 193 – 210.
Polls Pelaz M. and Graf J.-D. (1988), “Erythrocyte size as an indicator of ploidy level in Rana kl. esculenta before and after the metamorphosis,” Alytes, 7(2), 53 – 61.
Polls Pelaz M. and Graf J.-D. (1989), “Triploid all-male genealogies in a Rana lessonae — Rana kl. esculenta hybridogenetic population,” in: T. Halliday, J. Baker, and L. Hosie (eds.), Abstrs. of the First Congr. of Herpetol., September 11 – 19, 1989. University of Kent at Canterbury, UK, University of Kent, Canterbury [without pagination].
Ragghianti M., Guerrini F., Bucci S., Mancino G., Hotz H., Uzzell T., and Guex G.-D. (1995), “Molecular characterization of a centromeric satellite DNA in the hemiclonal hybrid frog Rana esculenta and parental species,” Chromosome Res., 3(8), 497 – 506.
Regnier V. and Neveu A. (1986), “Structures spécifiques des peuplements en grenouilles du complexe Rana esculenta de divers milieux de l’Ouest de la France,” Acta Oecol./ Oecol. Appl., 7(1), 3 – 26.
Rosanov J. M. and Vinogradov A. E. (1998), “Precise DNA cytometry: investigation of individual variability in animal genome size,” Tsitologiya [in Russian], 40(8 9), 792 – 800.
Rybacki M. (1995a [1994]), “Water frogs (Rana esculenta complex) of the Bornholm Island, Denmark,” Zool. Polon., 39(3 – 4), 331 – 344.
Rybacki M. (1995b [1994]), “Structure of water frog populations (Rana esculenta complex) of the Wolin Island, Poland,” Zool. Polon., 39(3 – 4), 345 – 364.
Rybacki M. (1995c [1994]), “Diploid males of Rana esculenta from natural populations in Poland producing diploid spermatozoa,” Zool. Polon., 39(3 – 4), 517 – 518.
Rybacki M. (1995d [1994]), “Pure populations of a hybrid Rana esculenta from German-Polish Usedom Island,” Zool. Polon., 39(3 – 4), 519 – 520.
Rybacki M. (1995e [1994]), “Structure and reproduction of water frog populations, predominantly RLL triploids, from Wysoka Kamieñska, Poland,” Zool. Polon., 39(3 – 4), 521 – 522.
Rybacki M. and Berger L. (2001), “Types of water frog populations (Rana esculenta complex) in Poland,” Mitt. Mus. Naturkunde Berlin. Zool. Reihe, 77(1), 51 – 57.
Schmeller D., Crivelli A., and Veith M. (2001), “Is triploidy indisputably determinable in hybridogenetic hybrids by planimetric analyses of erythrocytes?,” Mitt. Mus. Naturkunde Berlin. Zool. Reihe, 77(1), 71 – 77.
Schneider H. and Tunner H. (1982), “Struktur des Paarungsrufes und der Revierrufe bei triploiden Teichfröschen (Rana esculenta) (Amphibia: Salientia: Ranidae),” Salamandra, 18(1/2), 1 – 8.
Schröer T. (1996), “Morphologie und Ploidiegrade von Wasserfröschen aus unterschiedlichen Populationssystemen in Nordost-Polen,” Z. Feldherpetol., 3, 133 – 150.
Schröer T. (1997), “Lassen sich Wasserfrõsche phänotypisch bestimmen? Eine Feld- und Laborstudie an 765 Wasserfrõschen aus Westfalen,” Z. Feldherpetol., 4, 37 – 54.
Souèek Z., Kolman P., and Zavadil V. (1993), “Rozšíøení zab ve støedních Èechách III — vodní skokani (Rana esculenta synklepton),” Bohemia Centralis, 22, 7 – 34.
Terentjev P. V. (1927), “Miscellanea Herpeto-Batrachologica. VI. Bemerkungen über die Systematik und Verbreitung der grünen Frösche,” Zool. Anz., 74(1/4), 82 – 88.
Terentjev P. V. and Chernov S. A. (1949), Guide Book to Reptiles and Amphibians. 3rd Edition [in Russian], Sovetskaya Nauka, Moscow.
Tunner H. and Dobrowsky M.-T. (1976), “Zur morphologischen, serologischen und enzymologischen Differenzierung von Rana lessonae und der hybridogenetischen Rana esculenta aus dem Seewinkel und dem Neusiedlersee (Österreich, Burgenland),” Zool. Anz., 197(1/2), 6 – 22.
Tunner H. G. and Heppich-Tunner S. (1991), Genome exclusion and two strategies of chromosome duplication in oogenesis of a hybrid frog,” Naturwissenschaften, 78, 32 – 34.
Tunner H. G. and Heppich-Tunner S. (1992), “A new population system of water frogs discovered in Hungary,” in: Z. Korsós and I. Kiss (eds.), Proc. of the 6th Ord. General Meeting of the Soc. Eur. Herpetol. August 19 – 23, 1991. Budapest, Hungary. Budapest, pp. 453 – 460.
Uzzell T. (1963), “Natural triploidy in salamanders related to Ambystoma jeffersonianum,” Science, 139(3550), 113 – 115.
Uzzell T. and Berger L. (1975), “Electrophoretic phenotypes of Rana ridibunda, Rana lessonae, and their hybridogenetic associate, Rana esculenta,” Proc. Acad. Natur. Sci. Philadelphia, 127(2), 13 – 24.
Uzzell T., Berger L., and Günther R. (1977), “Diploid and triploid progeny from a diploid frmale of Rana esculenta (Amphibia, Salientia),” Proc. Acad. Nat. Sci. Philadelphia, 127(11), 89 – 91.
Uzzell T., Günther R., and Berger L. (1977), “Rana ridibunda and Rana esculenta: a leaky hybridogenetic system (Amphibia, Salientia),” Proc. Acad. Nat. Sci. Philadelphia, 128(9), 147 – 171.
Uzzell T., Hotz H., and Berger L. (1980), “Genome exclusion in gametogenesis by an interspecific Rana hybrid: evidence from electrophoresis of individual oocytes,” J. Exp. Zool., 214(3), 251 – 259.
Vedmederja V. I. (1984), “Some data on frogs of the genus Rana in Kharkov Province (based on the materials of the Museum of Nature, Kharkov State University),” Vestnik Khark. Univ. [in Russian], 262, 99 – 101.
Vinogradov A. E., Borkin L. J., Günther R., and Rosanov J. M. (1990), “Genome elimination in diploid and triploid Rana esculenta males: cytological evidence from DNA flow cytometry,” Genome, 33(5), 619 – 627.
Vinogradov A. E., Borkin L. J., Günther R., and Rosanov J. M. (1991), “Two germ cell lineages with genomes of different species in one and the same animal,” Hereditas, 114(3), 245 – 251.
Vinogradov A. E., Rosanov J. M., Caune I. A., and Borkin L. J. (1988), “Elimination of one of the parental genomes in the hybridogenetic species Rana esculenta prior to premeiotic DNA synthesis,” Tsitologiya [in Russian], 30(6), 691 – 698.
Wickbom T. (1945), “Cytological studies on Dipnoi, Urodela, Anura and Emys,” Hereditas, 31(3 – 4), 241 – 346. Zavadil V. (1995 [1994]), “On the distribution of water frogs (Rana esculenta synklepton) in the Czech Republic with some notes from this territory,” Zool. Polon., 39(3 – 4), 425 – 439.
Zavadil V., Kotlík P., Kolman P., and Maøík J. (1999), “Water frogs of District Cheb (West Bohemia, Czech Republic),” in: D. Schmeller and J. Plötner (eds.), , Abstrs. of the III Int. Symp. “Genetics, Systematics and Ecology of Western Palearctic Water Frogs,” October 11 – 15, 1999, Johannes Gutenberg Universität Mainz, Mainz, p. 23.